

Examples of how to calculate quantum numbers – l……0…….+l (values from negative l to positive l) (n-1) values from 0 and above only) no negative values. Numberġ,2,3,4 (values from 1 and above….) no negative values. The following table is useful in guiding you on how to calculate quantum numbers. Therefore, electrons can have either of the two values. The spin of an electron can either be +1/2 or -1/2. We know that an orbital can accommodate a maximum of two electrons, and they must have opposite spins.
This is used to represent the spin of an electron in an orbital. The electron spin quantum number is represented by the formula ms. This means that it takes values from negative l to positive l. The formula for magnetic quantum numbers depends on the angular quantum numbers. It represents the orientation of orbitals within a subshell. The magnetic quantum number is represented by the letter ml. The values of angular momentum (l) can be from 0, 1,2,3,4-(n-1). The value of angular momentum depends on the principle quantum number in that L= n-1.

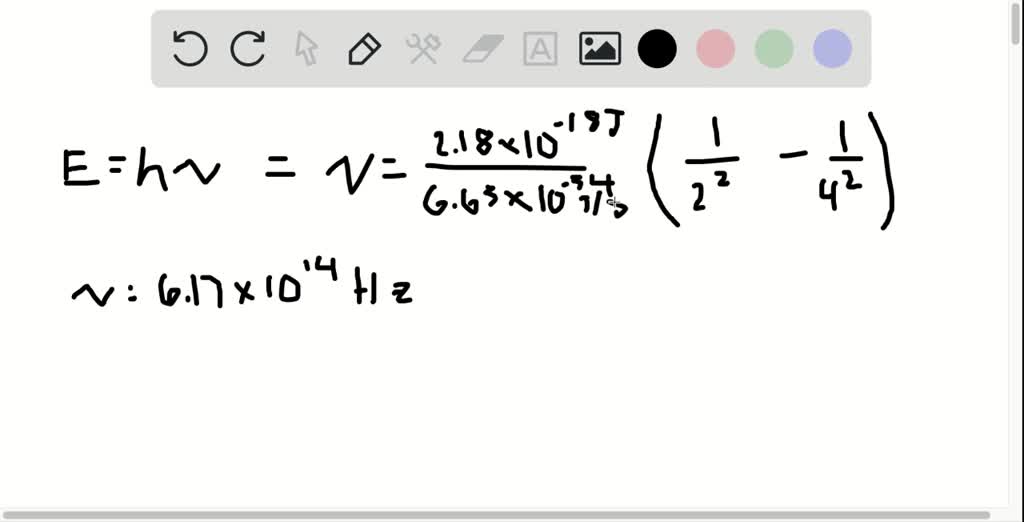
It represents the orbital to which the electron belongs. Angular momentum quantum number ( l)Īngular momentum is denoted by a letter (l). This is the process that describes spectrophotometry. During excitation, an electron absorbs a certain amount of energy that is enough to move it to the excited state, while during relaxation, the same amount of energy is released.ĭuring the relaxation of an electron, they release a certain amount of energy in a specific wavelength that is associated with a certain color. This process is called relaxation if the electrons move from a higher to a lower energy level, such as n=3 to n=2. If sufficient energy is applied, the electron will move a lower energy level to a lower energy level, such as from n=1 to n=2, and this process is called absorption. However, they can move from a ground state (lover n) to an excited state (higher n). The values of n can only be positive integers because the lowest energy level for every atom is 1.Įlectrons are usually localized in their energy level unless energy is applied. It represents the energy level to which an electron is located within an atom. The principle quantum number is represented by the letter n. These quantum numbers include: Principle quantum numbers (n ) There can never be a situation where 2 electrons have the same quantum numbers. There are usually four quantum numbers in which each electron in an atom has different quantum numbers. Quantum numbers are a set of numbers used to describe the characteristics of every electron in an atom. Examples of how to calculate quantum numbers.


 0 kommentar(er)
0 kommentar(er)
